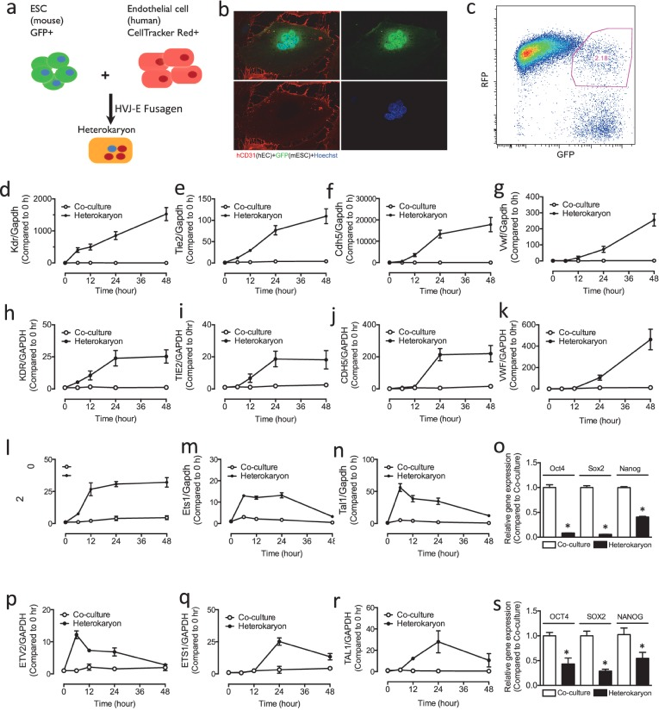
2. Nanotechnology and Vascular Biology (納米科技與心血管生物學)
E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis
Nanoparticles have emerged as a promising vector for miR delivery, and polyethylene glycol-polyethyleneimine (PEG/PEI) nanoparticles have been validated as an efficient delivery vector. However, rapid clearance and poor targeting limit their clinical value. We developed and tested a porous silicon multistage vector (MSV) delivery system, which is a micrometer-sized nanoporous microparticle. Large amounts of therapeutic agents have been packaged into PEG/PEI nanoparticles and loaded into the nanopores within the MSV microparticles.
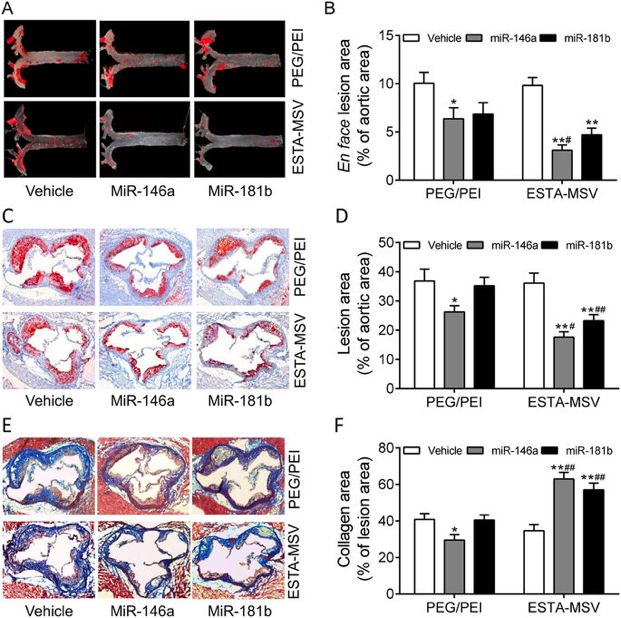
E-selectin is a surface marker of endothelial cell (EC) inflammation, one of the hallmarks of atherogenesis. Thus, we tested the hypothesis that delivery of microRNA (miR)-146a and miR-181b with an E-selectin-targeting multistage vector (ESTA-MSV) to inflamed endothelium covering atherosclerotic plaques inhibits atherosclerosis. Cy5-conjugated miR-146a and miR-181b were packaged in polyethylene glycol-polyethyleneimine (PEG/PEI) nanoparticles and loaded into ESTA-MSV microparticles. Both miRs were downregulated in tumor necrosis factor (TNF)-α-treated ECs. Transfection of TNF-α-treated mouse aortas and cultured ECs with miRs was more efficient with ESTA-MSV than with the PEG/PEI. Likewise, miR-146a/-181b packaged in ESTA-MSV efficiently suppressed the chemokines, CCL2, CCL5, CCL8, and CXCL9, and monocyte adhesion to ECs. Complementary in vivo tests were conducted in male apolipoprotein E-deficient mice fed a Western diet and injected intravenously with the particles prepared as above biweekly for 12 weeks. Treatment with miRs packaged in ESTA-MSV but not in PEG/PEI reduced atherosclerotic plaque size. Concurrently, vascular inflammation markers, including macrophages in aortic root lesions and chemokine expression in aortic tissues were reduced while the vascular smooth muscle cells and collagen increased in plaques from ESTA-MSV/miRs-treated vs. vehicle-treated mice. Our data supported our hypothesis that ESTA-MSV microparticle-mediated delivery of miR-146a/-181b ameliorates endothelial inflammation and atherosclerosis. This novel finding has just been published in Sci Rep (2016; 6: 22910.). [Pudmed] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783714/
Representative Figure 4: Effects of PEG/PEI/miRs and ESTA-MSV/miRs on the aortic atherosclerosis of ApoE−/− mice.