|
�ĤQ���G�Ī� - �����L�� (Oseltamivir)
[
back ]
²��
(���s��:
�K�O����\��)
�����L��(Oseltamivir)�O�@�ئ��ĤΦ���ܩʪ��J������A��w�����y��P�_�үf�r(influenza
viruses A)�άy��P�_�A�f�r(influenza viruses B)�����g�i�J(neuraminidase)�����D�ʡC���g�i�J(neuraminidase)�t�d���_��f�r
(virion)�W����G��(sialic acid)�Ψ�ݾl���A�åB��U�������X���f�r��C���f�r�챵IJ������L��(Oseltamivir)��A���ķ|�Ϩ�f�r��E�X�b�H�D�ӭM�������C�o���Ī��|��C�f�r�b�H�����c���Ǽ�����O(McNicholl
2001)�A�q�ӹF�ܭ��C�f�r�P�V�O�C
�����L��(Oseltamivir)�{�b�D�n�Χ@��w���y��P�_�A�Ϊv���@��²���ʷP�_�x���C�ҿ�²��P�_�x���O���@���H�W�w�̬V�f�ɶ������G�ѡCH5N1�f�r������L��(Oseltamivir)�Q���ӷP�A���q���������ܨ㦳�{�ɪv���į�C
�{�ɬ�s��ܡA�p�x���X�{����48�p�ɡAneuraminidase�J�����Ĵ�ַP�_�x��������ɶ��A�Ҧp�٦ĵh�B�o�����Y�h�i��֬�0.7-1��C�{�ɮį�i�F60-70%�C�p�G���x���X�{����30�p�ɤ��o���w�̡A��v�����ġC�t�~�A�b��B��������L��(Oseltamivir)�b�ӭM��f�r�P�V���K�̤������A�]�S���X�{�t���@��(Burger
2000)�C
�t�~�A�����i�������L��(Oseltamivir)�i��|�ޭP���L�G�z�D���A���t�@��(Doucette
2001)�C�̪�A�����L��(Oseltamivir)��Q�o�λP�X�v�믫���դΨ�W�饻�C�~�۱��C���L�A�{�b�S���Ҿګ��A�ζ����L��(Oseltamivir)�M�۱��ɦV���]�G���Y�C
�ƾǵ��c
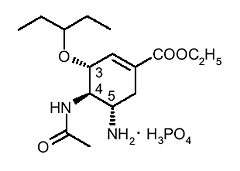
�����L��(Oseltamivir)�O�A������e��(prodrug)�A�O�ݭn�z�L��ѧ@����Ʀ����ʧΦ��A�����L��(Oseltamivir)�n��-4-�L �i-5-���-3-(1-ethylpropoxy)-1-���A�J-1-�n���C���Q
(carboxylate[3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate
phosphate.) �C�����L��(Oseltamivir)�Ī��]�p���o�{�O�Q��x�����R������G��(sialic
acid)�P�y��P�_�үf�r���ʰϤ�neuraminidase�J�����E�X������o�X�ҥ� (Lew
2000)�C�����L��(Oseltamivir)���s���O�z�L����sialic acid�������c(�]�A�[�W�ݪo����lipophilic
side chain)�A�ت�����Ω�f�A(Kim
1998)�C�������ƾǵ��c�{���p�U�G �i-5-���-3-(1-ethylpropoxy)-1-���A�J-1-�n���C���Q
(carboxylate[3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate
phosphate.) �C�����L��(Oseltamivir)�Ī��]�p���o�{�O�Q��x�����R������G��(sialic
acid)�P�y��P�_�үf�r���ʰϤ�neuraminidase�J�����E�X������o�X�ҥ� (Lew
2000)�C�����L��(Oseltamivir)���s���O�z�L����sialic acid�������c(�]�A�[�W�ݪo����lipophilic
side chain)�A�ت�����Ω�f�A(Kim
1998)�C�������ƾǵ��c�{���p�U�G
�b�̪�ɴ��A�����L��(Oseltamivir)�Ψ䬡�ʥN�ª��A�Q�٤���GS4104�BRo
64-0796�BGS4071��Ro 64-0802�C
�Ī��N�°ʤO��
�z�L�f�A�~�|�A�����L��(Oseltamivir)�ܧַ|�Q�G�z�D�l���A�M��b�xŦ��Ƭ����ʶ����L��(Oseltamivir)�n��N�ª��A�̫�|���G�������A�]�A�W�ΤU�I�l�D(Doucette
2001)�C�p�G�q�W�z�~�|�A�������ʶ����L��(Oseltamivir)�N�ª����ͪ��į�ʥi�F80%�C�W�A30�������N�i�P���G���������L��(Oseltamivir)�N�ª��A�Ө�̰��@�O�j��3��4�p�ɤ���C���ӡA��N�ª��@�ץH�b�I��6��10�p�ɤ@���h��(He
1999)�C
�̲צ�߱ưh�b�I���A�H���d���H���Ҥj��1.8�p�ɡC���w���Ƿl�`���f�H�ӻ��A�N�ª��M���v�P�ٻ���(creatinine)
�M���v�O�����C�A�Y�O���f�A�Ī���23�p�ɡA�ٮ�����M���v<30�@��/����(Doucette
2001)�C�G���A�����y�f�H�A�ξ��q�A��֦�79�@�J�C��@���A�F�ܦٮ�����M���v<30�@��/����(1.8��/�p��)(He
1999)�C
�߳J���H�X��3%�C�z�L�Ǥp�y�L�o�ά��ʺު����c��j�@�ΡA�N�Ī��Ψ䬡�ʥN�ª��ƪn�X��~�A�A�S���~��s���N�¶i��C�ƦX���J�S���P�ӭM���P450�V�X�\���ƻï�cytochrome
P450 mixed-function oxidases��glucuronosyltransferases
���ͤ���(He
1999)�C�]���A�Ī��P�Ī��ۤ��@�Ϊ��i��O�ܧC�A���C�F��Ǥp�ޥ~���ӭM�t���l��e���ƪn�����j�C�t�~�AProbenecid���j�F��Ŧ�ƪn�����L��(Oseltamivir)����O(Hill
2002)�C���M�o�ر��p������L��(Oseltamivir)�{�����ΤW���I���Q�A��Probenecid�Υi�ȸѨM�����L��(Oseltamivir)�s�q�u�ʰ��D(Butler
2005)�C
�w���x�l�`���f�H���������L��(Oseltamivir)���N�¨èS���M�ΡA���ݭn�վ㾯�q(Snell
2005)�C
���~�ѤH�h�譱�A���ʥN�ª��b�餺������ɶ���~�C�H����25%�C�����ݭn�վ㾯�q
(He
1999)�C
�ѩ�j��1��12���p��������L��(Oseltamivir)�N�²v���C�~�Φ��H����,
�]���Ī��b���餺���ɶ����u�C���q�譱�A�p���n�W�[��2�@��/����2��@���A�~�۵��H�����`���q(Oo
2001)�C�j��1�����k�����i�H���Ħa�N�¤αƪn�X�����L��(Oseltamivir)(Oo,
2003)�C�ӹ����~���ൣ�ӻ��A�A�ζ����L��(Oseltamivir)�ɥi��ǸT��(���r��)�C
�r��
�̱`�����Ƨ@�άO���L���ߤιæR�A�h����A�ī᪺1-2�ѥX�{�C
�H�W���}�������O�����L��(Oseltamivir)���X������ұ����D�o�ӡC�t�~�A�b��L��D���A�礣���O�_�P�A�ζ����L��(Oseltamivir)�����C�p�G
- �X�l�B�����Φ��~�ȡB�r�ʪ����a�����ѯg(toxic epidermal necrolysis,
TEN)
- �x���B�x�\��X�{�����`
- �߫ߤ���
- ���z�B���Ӥ��M
- �[�t�}���f�c��
�A�ζ����L��(Oseltamivir)���Ӥ��|�W�[�X�{�ֽ����������|(Nordstrom
2004)�C�t�~�A���ǧ{�����i���X�i��ް_�S���ʥֽ������A�|�Ҧ��G��x�w�Ưf�H�Ӷi��xŦ������N��A�A�Χ@���w���Ķ����L��(Oseltamivir)
�� zanamivir�ӥX�{�������l(Kaji
2005)�CŲ��H�W�ƥ�A�������Ī��z��(FDA)�n�D�����L��(Oseltamivir)���~���ҤW���w�[�W�u�i�ް_�Y���ֽ�/�L�Ӥ����v�C�p�G�A�Ϊ̸U�@�X�{�Y�����l���p�A���ߨ谱��A�Ωα�IJ����(FDA
2005)�C
1���H�U���ൣ����ij�A�ζ����L��(Oseltamivir)�A�]�������L��(Oseltamivir)�葉���i����礤�X�{�L���r�����C�t�~�A�p�G���q�F1000�@�J/��������L��(Oseltamivir)�C���Q(�j���O�ൣ��ij���q250��)�A�|�X�{�j�q�����L��(Oseltamivir)�C���Q�n�s��j���C�ƹ�W�A�ൣ�������L��(Oseltamivir)�C���Q�b���n�s�����p�O�����H1500���C�Ӷ����L��(Oseltamivir)���q�������v�T�N�S�������{�ɤ��R�C���������L��(Oseltamivir)������������帣�̻�(blood-brain
barrier)���Ҽv�T�A�G�@�볣����ij��1���H�U�ൣ�A�Φ��ġC(�ɥR:�@��1���ൣ�帣�̻٤w�ͦ����C)
�����L��(Oseltamivir)�ݥ�������C�Ī��A�ҥH�i��便���έL��@�w���v�T�C����s��ܡA�����L��(Oseltamivir)������X�{��¨Ŧѹ����ŲG�A���N�S���b�H�餤���L��������A�G����֩w�����L��(Oseltamivir)�O�_��q�H�����ŲG���ƥX�C
������f�H�A�ζ����L��(Oseltamivir)�ӥX�{�믫���`�����i�A�饻�����w�N�f�H�X�{�믫���`�������A�ζ����L��(Oseltamivir)���Ƨ@�ΡC
�\��
�v��
�����L��(Oseltamivir)�@��ζq��75�@�J�C��2���A����5�ѡC���]�y�P�ӵo�N�f�H�A�o�f�ɶ�����1.5�Ѫ��f�H�A�i��ֱw�f�ɶ���1.5�Ѥδ�Y����38%
(Treanor
2000)�C�p�G��b�o�f12�p�ɤ��A�������{�A�w�f�ɶ���i���3�ѡC�`���A�V�����������L��(Oseltamivir)�A�V����ֵo�N�ɶ��μx���Y���{�שM��_���`���ɶ�
(Aoki
2003)�C
�@������o�N�f�H�A����?������39�XC(Kawai
2005)�C�����L��(Oseltamivir)�ĮĤj���A��24�p�ɤ�����(Nichson
2000)�C�q�]�A10�Ӧw��������������礤���ΦX���Rmeta analysis�A�o�{Oseltamivir�i��C�w�U�I�l�D�ֵo�g(Kaiser
2003)�C
���w���C�ʩI�l���x�e�f���w�̡A�]�A�C�ʤ��ު��B����ʪͮ�~�B���ޭ��ݩΤ����X�i�B�κC�ʤ�Ŧ�f�������L��(Oseltamivir)���v���\�ĤΦw���ʫh�|�����T�C�b�@�Ǥp���H�����դ��A�����L��(Oseltamivir)�禳�����C�w�ֵo�g���|(11%
vs 45%)�A�ΥΧܥͯ����ݭn(37% vs 69%) (�Ƶ�: �e�̬������v���աF��̬�����)
(Lin
2006)�C
�����L��(Oseltamivir)�v����y��P�_�A�f�r(influenza
viruses B)���į��y��P�_�үf�r(influenza viruses A)���C�C(������H5N1�f�r�ĮĬ�s���U��)�C
�q�g�پǨ��פW�A�]�A�Ҽ{�y��f�Ǹ�ƤΧܯf�r�Ī��{�ɸ���A���O���@�ǵL�̭]�`�g�ΰ��M�̭]�`�g�f�H�i����R�C�`�O���ζ����L��(Oseltamivir)�v���O�����ŦX�g�ٮįq�C�Ө�L�f�H�A�ݵ��ݧֳt���յ��G�~�}�l�v��(Rothberg
2003)�C
�w��
�b�i������L��(Oseltamivir)��w�̤������դ��A�����L��(Oseltamivir)���w���O���ֱw�̼ƥ�[8/12
(67%)�b�w�����դ�8/21(38%)�b�����L��(Oseltamivir)�A�β�]�F�δ�֤ް_�P�V�ʩI�l�D�e�f(4/12
vs�A0/21�Fp=0.16�F�į�61%)(Hayden
1999a)�C�o�ǼƾڬO�q1559���d�εL�K�̤��������H(18��65��)�i��F���P�����ձo�X�ӡC�L�̤�����աG�����L��(Oseltamivir)�A�β�(75�@�J��150�@�J�C��)�Φw������(Hayden
1999b)�C�A�ζ����L��(Oseltamivir)(1. 2%)�@�չ�w�y�P���|��_�A�Φw�����t�@�ո��C(4.8%)�C�����L��(Oseltamivir)���w���y�P�ĪG�F74%(Hayden
1999b)�C�βΦX���R��k���R�C�عw�����դ��A�����L��(Oseltamivir)���w���y�P��O�F70��90%(Cooper
2003)�C
���w���a�~��IJ���y�P���ЭӮ�(IC)�A�o�{�����L��(Oseltamivir)��Ĺw���y�P�F89%(Welliver
2001)�C�b�@���H�����դ��A�A�Φw�����զ�12.6%(26/206)�X�{�y�P�x���A�ۤϪA�ζ����L��(Oseltamivir)�եu��1.4%(3/209)�C�b�t�@�H�����դ��A�a�~��IJ�H�b���y�P���ЭӮסA�Y�X�{�����y�P�x��(�p��Ű��L37.8�XC�Ϋy��)�ɫh�H��������աG�@������i��Q�Ѫ���IJ�f�r��w���v��(post-exposure
prophylaxis�APEP)�t�@�իh�o�f��~�v���C�Ҧ����ЭӮh�������Ѫv��(Hayden
2004)�A�����L��(Oseltamivir)�w���v��PEP�68%�w���v�C�����v�������ЭӮסA�A�Φw�����զ�13%(33/258)�X�{�y�P�x���A�ۤϪA�ζ����L��(Oseltamivir)�եu��4%(10/244)
(p=0017)�C
�q�g�پǨ��פW�A�A�ζ����L��(Oseltamivir)�w���y�P��_�A��amantadine�Τ��@����w����ŦX�g�ٮįq(Risebrough
2005)�C���O�t�~�@�Ӫ�����ΦX���R�A�{�������L��(Oseltamivir)�����Y�����ǬV�ʩάy��ʪ��P�_�f��������\�ġA�����u�`�ʷP�_�f�N���ө���(Jefferson
2006)�C
��ܩʯf�H�ոs
�z�L�����Φw������k�A��548��z�Φ~�Ѫ��b�a�H�h(����Ƭ�81���A�h��80%���K�̪`�g)�i��f�A�����L��(Oseltamivir)�w���y�P����(Peters
2001)�C���A�Φw�����A�����L��(Oseltamivir)���C92%�H�h�w�y�P���|(1/276
= 0.4 % �F12/272 = 4.4 %)�C�ӥB�����L��(Oseltamivir)��Ħa��C�w�y�P�~��ֵo�g(Peters
2001)�C
�ൣ�G���f�H�f�A�����L��(Oseltamivir)��A���ֱw�y�P�ɶ���36�p�ɤΨ�L�X�{�x���ɶ�(�p�y�¡B�o�N��)�C�[�W�A���֪�44%�w���ժ����|�ΪA�Χܥ;�������
(Whitley
2001)�C�b�̪��s���A�o�{�����L��(Oseltamivir)���Y�u���ݨൣ�X�{�x���ɶ��B�ﵽ��ͥ\��H�δ�C���ݴc�ƾ��|(51
% �� 68 %) (Johnston
2005)�C
�ܩ�A�����L��(Oseltamivir)���C�ʤ�Ŧ�e�f�ΩI�l�D�e�f���\�Ĥ����T�w�C�t�~�A�L�����ܡA�]�i������L��(Oseltamivir)�v���ӥX�{�Y�����p�A�ݰe�|���e�C�٦��A�f�H���L���貾�Ӥ�N�����Ӥ��A�]�̭]���K�̤O�ӧC�ӱĥζ����L��(Oseltamivir)�@�ιw������(Machado
2004)�C
��H5N1�V�y�P���į�
�b�պ��礤�A�����L��(Oseltamivir)���T��y��P�_�ҤΤA�f�r(influenza
viruses A & B)���@�w���ܯf�r��O�A�]�A���@�צb����v�h��H5N1 �� H9N2�f�r(Leneva
2000)�C�ھ�WTO��Ʃұo�AH5N1�V�y�P�Ӯפw���(Writing
Committee of the WHO 2005)�C�ۤϡA�����������ܶ����L��(Oseltamivir)��Ĵ��H5N1�f�r�P�V�C�̪�A���i���X�Y�ϾA�����q�A�]�������f�r�ۧڽƻs�A�Ʀܤް_���Ĥ���(de
Jong 2005)�C����D�A�O�_���[�����q�Ω����v���ɶ����b���פ��C�t�@�ӰQ�װ��D�A�����L��(Oseltamivir)��A�Ω����f�r�w�i��ƻs�o�ӱ��p�C�{�u�o�Ƿ������Ҿڻ�������ĥζ����L��(Oseltamivir)���֯f�r�b�餺�ƶq�A�H�μW�[�f�H�s���v(de
Jong 2005)�C�o�Ӽƾڵ��G�A�P�����ѹ�����O�@�P���C�Ҧp�i��5�����{���ܡA����ѹ����s���v�F50%�F�i��8�����{���ܡA����ѹ����s���v�F80%
(Yen
2005b)�C�t�~�@�ӹ���A�Y�ϩ���F5�Ѷi�����{�A��s���v�]�i��0%�ɦ�75%
(McCullers
2004)�C
���������L��(Oseltamivir)���q�]���H��w���C�����ܡA5�����{150�@�J�C�ѤG����6�P���{75�@�J�C�ѤG���@�w�����ΡA�]�i��O�\�i�����q(Ward
2005)�C
��1918�~�y��P�_�f�r���į�
�o�ص��X�ʯf�r�O��1918 NA��1918 HA��1918
NA��Ӥ�k�c���C�Ӷ����L��(Oseltamivir)�����������b�ӭM��´���i����Φѹ��������X�ʯf�r�C�]���A�{�������L��(Oseltamivir)�����1918�~�y�檺�P�_�f�r�Ψ��������f�r(Tumpey
2002)�C
��ܩ�
�b�պ��礤�ANA����E119V, R292K, H274Y,
and R152K�|�ް_������L��(Oseltamivir)���ĩ�(McKimm-Breschkin
2003)�C�ӯf�r��R292K���ܪ��ܡA��ۧڽƻs��O�|�ॢ�A�Ψ�P�f��O�b����ѹ����ͫ��C��10,000��(Tai
1998)�C�P�˦Ө��A����H274Y���ܪ��ܡA��ۧڽƻs��O�|���3��(logs)
(Ives
2002)�A�G�ݦh��100���f�r�ƶq�~�i�ϥ��^�P�f�A�ͫ��Ǽ��O�C(Herlocher
2004)�C
�ھڥH�W�����A�f�r�X�{���ܦӼv�T���A����O���ܡA�ڥ����ݭn���`�C�̪�A���dz��i���H5N1�f�r��ް_������L��(Oseltamivir)���ק��ĩʳo�����k(Le
2005, de
Jong 2005)�C���M�Y�ϪA�ξA�������L��(Oseltamivir)���q(���f�x����@�ѪA��)�A�������f�r�ۧڽƻs�Τް_���Ī��f�r�A���������D�u����]�O�_�ѩ�ɭP�f�r�j�q�ƻs�έ��g�f�H�餺���IJz�����w�����ܡC
�Ϥ��A�u�`��H1N1��H3N2�y�P�f�r�b���H�Φ~�C�H������ܦ]�l��O���C�A�h����ൣ�C��@����s���X�A�f�r�����g�i�J(neuraminidase)���ܬ�9/50�f�H(18%)(Kiso
2004) �C�Ӧ]���ൣ�O��f�r�䤤�@�ӶǼ��C���A�Y�Ϥ��ѫᱵ���v���A�o�Ǹ�Ƥ]�ݽլd�C
�b�����s���A�i�o�{�ܶ����L��(Oseltamivir)���ܲ��ؤΧ�Zanamivir���ܲ��ط|�o�ͥ�e��ܩ�(Cross-resistance)�C�䤤��Ө������L��(Oseltamivir)�ް_����(E119V,
H274Y and R292K)�|�b���g�i�J(neuraminidase)�P�@���i��ľl���A�X�{��䤤��Ө�Zanamivir�ް_���ܪ��f�r��(E119G/A/D,
R152K and R292K) (Tamiflu
2005)�C
�Ī��ۤ��@��
�ھ��IJz�Ǥ��Ī��ʤO�Ǭ�s��ܡA�Ī��v���W�X�{�����L��(Oseltamivir)�P�S�������L��(Oseltamivir)�ۤ��@��
(Tamiflu 2005)�C�N�����L��(Oseltamivir)(�ζ����L��(Oseltamivir)�n��)�ä��O�βӭM���P450�����Χ���C
�Ϊk��ij
�ڷ�
�����L��(Oseltamivir)(�S�Ӻ�Tamiflu?)�w���ڷ������\�i�C�Ϊk�����ξ��q���ھڬ��ꥫ���{�i�C
����
�����L��(Oseltamivir)�Χ@�v���@��²���ʷP�_�x���C�ҿ�²��P�_�x���O���@���H�W�w�̥X�{�x���f�ɶ������G�ѡC�A�̡A�����L��(Oseltamivir),�A�Ω�@���H�W�w�̹w���y�P�C
�@����w�v�����q�A13���ΥH�W�H�h��75�@�J�C�ѤG���A�H5�Ѥ@�����{�C�p����]�A���n���ൣ�Φ��H�A�i���30�B45��60�@�J�f�A�IJG�C�ѤG���C
��ij���q�G
|
�魫
|
5�ѫ�ij���q
|
| ≤15 ����(≤ 33 �S) |
30�@�J�C�ѤG��
|
| > 15����to 23����(> 33 �S�� 51 �S) |
45�@�J�C�ѤG��
|
| > 23 ����to 40 ����(> 51 �S�� 88
�S) |
60�@�J�C�ѤG��
|
| > 40 ���� (> 88 �S) |
75�@�J�C�ѤG��
|
8���H�W�ൣ�p��]�A�Ī��i��75�@�J���n�t��C
�w���譱�A�@���ij���q75�@�J�C��@�ѡA�����ܤ֤C��C���@���ΥH�W���f�H���f�A�����L��(Oseltamivir)���q���p�U�G
|
�魫
|
7�ѫ�ij���q
|
| ≤15 ����(≤ 33 �S) |
30�@�J�C�ѤG��
|
| > 15����to 23����(> 33 �S�� 51 �S) |
45�@�J�C�ѤG��
|
| > 23 ����to 40 ����(> 51 �S�� 88
�S) |
60�@�J�C�ѤG��
|
| > 40 ���� (> 88 �S) |
75�@�J�C�ѤG��
|
�`��
�����L��(Oseltamivir)�O�@��,���ܩʯ��g�i�J���(neuraminidase
inhibitor)�C�����v���ɶ��@�w�n�b�X�{�x����48�p�ɤ��A���̦n���M�O���֩Φb24�p�ɤ��C�@��H������L��(Oseltamivir)���@�ĤO�C
��a������ij�����N�����L��(Oseltamivir)���N�������̭]�C
���H5N1�f�r�A�����L��(Oseltamivir)���\�ġB�̨ξ��q�Υ������{�����ݪ֩w�C
�ӫ~�W�١GTamiflu™
75�@�J���n (10�ɤ@��) �f�A���� (�Τ��ը�12�@�J/�@�ɡF�@�����t25�@���a�B�G)�C
�Ī������G���g�i�J���(neuraminidase
inhibitor)
�A�Ϋ��ޡG�A�Ω�@��D�����ʫ�ʬy�P�w��(1���H�W)�A�X�{�x��������ѡC
�Χ@�w���y�P�A�@��A�Ω�1���H�W���w�̡C
�зǾ��q(�Χ@�v��)�G75�@�J�C��⦸�A�������ѡC
���w�̩Χ]�A��ê�����H�w�̡A�i��Τf�A�G���C���q��ij�аѦҥH�W�C
�зǾ��q(�Χ@�w��)�G75�@�J�C��@���A�����ܤ֤C�ѡC
���w�̩Χ]�A��ê�����H�w�̡A�i��Τf�A�G���C���q��ij�аѦҥH�W�C
�S�����q�G���w�̨��M�ٻ����M���v(serum
creatinine clearance)�@�����G10-30�@��/�����h�A�Ϊv�����q��75�@�J�C��⦸�A�������ѡB�w�����q��75�@�J�j�ѩ�30�@�J�C�ѡC�w����A�Ω�i��`�W��G�z�R�θ����z�R�������ǯf�w�̫h�������T�����q�i��ij�C
�Ī��ʤO���G�f�A�����L��(Oseltamivir)�@��b�G�z�D�l���A�M����Ʀ������L��(Oseltamivir)�n��(carboxylate)�C�̫�A�����L��(Oseltamivir)�n��z�L���G�ƥX��~�A�@��b�I��(half-life)��6-10�p�ɡC
�T�ҡG�����L��(Oseltamivir)���A�Ω�1���H�U���y�P�w�̡C���D���Ĺ便�����Q�q�X�z�a����i��M�`�L����I�_�h���A��(�ݥ�������C�Ī�)�C
�Ī��ۤ��@�ΡG�������㪺�Ī��ۤ��@�ΡC
�Ƨ@�ΡG�@��Ƨ@�ηũM�Τ��ת��A�p�c�ߤιæR�A�h����A�ĭ���ѡC
�m��G���X�{�y�P�x����A�w�����ɦ��A���Ī��C�P�����b��IJ�f�r�����P�V�e�A�A���Ī��@�w���C
�p�Y��A�ĥi��u�ȩʸz�D���áC
��Ѧ~�w�̤��ݽվ㾯�q�C
�P�@�ɶ��i���ΪA�ġA���|���Ī��b�夤�̰��@�פ�AUC���v�T�C
�Ī��J�s�D�a��(��25�XC��77�X F)�A�u�ȶJ���i��15�X
to 30�X C (59�X to 86�X F)�C
�����L��(Oseltamivir)�f�A�G���A�̦n���ľ��v�ը��t�o���f�H�C
�J�s�f�A�G���A�ū�2�X to 8�X C (36�X to 46�X
F)�A���i�B�áC
�����L��(Oseltamivir)���i�N���y�P�̭]�A�w�������H��a�������ޱ����C�~�y�P�̭]�C
���p�����
�ڬw: http://influenzareport.com/link.php?id=14
����:
http://influenzareport.com/link.php?id=1
|

