|
�ĤQ���G�Ī� - �㨺�̭� (Zanamivir)
[
back ]
²��
(���s��:
�K�O����\��)
�㨺�̭�(Zanamivir)�O�@�ؤf�ħl�J�����C�{�b��19�Ӱ�a�]�ĥΥ����v���ιw���y��P�_�ҤΤA���f�r(influenza
viruses A & B)���ΡC�㨺�̭�(Zanamivir)�O���g�i�J�}�J�ժ��v���ʧ��(neuraminidase
glycoprotein)�C���g�i�J�}�J�զb�y�P�f�r�i��J�I�ɦ��ܭ��n�@�ΡC���P��G��(sialic
acid)���ۦ��A�O���g�i�J(neuraminidase)���۵M���(Varghese
1992, Varghese
1995)�C
�㨺�̭�(Zanamivir)�O�z�L�l�J�覡�A�����i�J�I�l�D�C�������@���p�⬰���g�i�J(neuraminidase)��50%����@��(IC50)��1,000���C�Ө���������10�r�����C
���h�åX�{�t�Ωʬy�P�P�V�A�S�O�O�̪H���P�V�V�y�P(de
Jong 2005)�A�㨺�̭�(Zanamivir)�i��N���O�A�X���Ī��C
�L�h��X�~�A�X�{�L�X�v�]�A�Τ㨺�̭�(Zanamivir)��A�X�{���j�ˡB��ݡB���l�C�¯l�ιL�Ӥ���(�p���Ϋ|����~)�C���L�A���F�X�v�u�����p�~�A�p�����A�Τ㨺�̭�(Zanamivir)�]�O�w����(Hayden
1997)�C
�P�@�ɶ��A�Τ㨺�̭�(Zanamivir)�Ϊ`�g�C���ʤT���y�P�̭](inactivated
trivalent influenza vaccine)�A�@�뤣�|��s�y�ܾ������(antihaemagglutinin
antibodies)���ͭt���v�T(Webster
1999)�C�o�ǫO�@�ʧ���h�b12�Ѥ�����(Cox
2001)�C
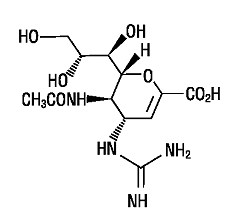
�ƾǵ��c
�㨺�̭�(Zanamivir)�ƾǦW�٬O5-(acetylamino)-4-[(aminoiminomethyl)-amino]-2,6-
anhydro-3,4,5-trideoxy-D-glycero-D-galacto-non-2-en
onic acid�C�������c�{���p�U�G
�Ī��ʤO��
�����ܡA��10-20%���㨺�̭�(Zanamivir)�|�z�L�f�ħl�J��F�ͳ��C�l�U���|�d�b�|��A�j��4-17%�Ķq�Q�H��l���C��10�@�J����A�b1-2�p�ɤ��A�Ī��b��G���F�̰��@�סC��J���H�X�Q����(<
10 %)�C�㨺�̭�(Zanamivir)�̫�q���G���ƥX�A��ӹL�{�b24�p�ɤ���(Cass
1999b)�C��b�I����2.5-5.1�p�ɡC
�������s���A���R�ߪ`�g�㨺�̭�(Zanamivir)��A�Ī������|���t��I�l�D�H�G�A�i�ӥi������y��P�_�үf�r(influenza
viruses A )�P�V(Calfee
1999)�C
�r��
�W�A�㨺�̭�(Zanamivir)�]�i��w���A��ް_�I�l�D���D���|�Q���C(Loughlin
2002)�C�b�պ��թΰʪ��餺���դ��A�㨺�̭�(Zanamivir)�t��@�r�ʫܧC�A�Y�ϥΤ`�h100�����q�A���|���������������r�����C�]�����I�l�D����E���{(Freund
1999)�C
�@���ij�㨺�̭�(Zanamivir)�����q�A�O���|�O�w���I�l�D�e�f���w�̥X�{�Ƨ@�ΡC���L�A���w�̾ں٪A�Τ㨺�̭�(Zanamivir)��A�X�{���j�ˤΪͥ\���C(FEV�A�Φy�p�I��̰��y�t)�C�Ӧ����D�Ӯפ��A�o�DZw�̥������Ǽ�b�I�l�D�e�f�A�p���ݩκC�ʪ���ʩI�l�D�e�f�C�ѩ�i��|�ް_�Y�����D�A�G���@�볣����ij����b�I�l�D�e�f���w�̪A�Τ㨺�̭�(Zanamivir)�C�@��w�̥X�{���j�ˤΪͰI�ܪ��ܡA���ߨ�A�Τ㨺�̭�(Zanamivir)�C�p�G�X�{�Y���x���A�����Y�ɶi��v�z�ΰe�|�[��C
�]���A�Τ㨺�̭�(Zanamivir)�A�X�L�Ӥ����]�A�|����~���Y���ֽ����l�A�O�Q���u���C�U�@�u���X�{�DZ��p�A���ߨ谱����ĤγB�z�ثe���p�C
�b�A�Τ㨺�̭�(Zanamivir)�դΪA�Ψſ}���w�����դ���A��̥X�{�Ƨ@�Ϊ����Ʈt���h���G�@�ˡC�Ƨ@�Υ]�A�G���m�B���ߡB�Y�w�B�Y�h�B���{�B���h�B�C�¯l���C�̦h�X�{���礣���`���O�b�ĤT���v����s�A�]�A�G�x�J���W�ɡBCPK�B�O�ڲy��֤ΧC�ݤ��ʲy(neutropenia)�C�P�ˡA�b����Ψſ}�w�����էO�ҥX�{��ʬy�P�x�������Ƥ]�t���h(Relenza
2003)�C
�ۤϡA���5-12���ൣ�ӻ��A�X�{�f�x(�㨺�̭�(Zanamivir)
20 %, �w����9 %)�B�y��(�㨺�̭�(Zanamivir) 16 %, �w����8
%)�Ϋ|�藍�A(�㨺�̭�(Zanamivir) 11%, �w����6%)�A�����F�A�Τ㨺�̭�(Zanamivir)�ް_�Ƨ@�Ϊ����|��A�Φw�������h�C�ӺC�ʩI�l�D�e�f�譱�A�X�{�U�I�l�D���}�����]�A���ݡB�y�©ίf�r�ʩI�l�D�P�V�������y�P�x���A�t���h����7�Ӥ㨺�̭�(Zanamivir)�A�Ϊ̤]�X�{�H�W���}�����A���12�Ӧw�����A�Ϊ̥u��5�ӥX�{�H�W���p�C
�H�U�����}�����ӮO�㨺�̭�(Zanamivir)���X������Ҧ����^�ӡC���O�������ƾڤάO�_�u���P�㨺�̭�(Zanamivir)�����A�{�����֩w(Relenza
2003)�G
- �L�Ӥ����A�]�A�|����~
- �߫ߥ��`�A �w��
- �|�ϩ��z
- ���j�ˡA���
�q�����L����������㨺�̭�(Zanamivir)������s�C�b�ʪ����礤�A�㨺�̭�(Zanamivir)������ɭP�y���Ψ�L���D�C
�b�j�ѹ����W�A�o�{�㨺�̭�(Zanamivir)��q�ŲG�ƥX�A�������b�H�鰵�L��������A�G���S�������ܤ㨺�̭�(Zanamivir)��b�H���ŲG���ƥX�C
�į�
�p�G�b�y�P�o�f��48�p�ɤ��l�J�㨺�̭�(Zanamivir)�A���֨�X�{�x���ɶ���2.5��C�㨺�̭�(Zanamivir)���n�B���G�b���ǯf���w��(�p����b�ΰ��M�e�f)�Φ~�ѱw��(≥50��)�����C�ۤϡA���@�ǩ����C�ũίf���������w�̡A�㨺�̭�(Zanamivir)�_���@�δN����j�C
�Ω�w���譱�A�H�ϥΦw��������A�㨺�̭�(Zanamivir)������֮a�x�X�{�y�P�x���A��i������i�|�X�{�y�P�ӮסC
�v��
������㨺�̭�(Zanamivir)�@�{�ɬ�s�A�O�b1994-1995�~�A�_��38���ߤμڬw32���ߡA�H�H�������������k�A��f�H�����@�X���R�C�b��s���A�j�����y�P�x���@�骺�ɶ�(4
vs. 5��) (Hayden
1997)�C����o���j�v���o�q�O�}�l���{�ɤw�X�{�Y���x�����w��(Monto
1999)�C�[��50?�H�W�w�̥i��3�����{�o�q�A���[��50���H�U�w�̫h�i��1�����{�o�q�C�o�{���M�w�̤��v���o�q��2.5��(Monto
1999)�C�[�W�A�㨺�̭�(Zanamivir)���e���X�{�y�P�ֵo�g���w�̤]���ġA�p�~��≥65���B�C�ʪ���ʩI�l�D�e�f�B��Ŧ�f�B�}���f�ΧK�̥\���ê(Lalezari
2001)�C
�y�P�P�V�@���ް_�I�l�D�ֵo�g�A�h�ĥΧܥͯ��v���C�H�ΦX���Rmeta-analysis��k�i��7���{�ɴ��աA�]�ϥΦw������ӻݧܥͯ��v���ֵo�g(�]�A��ʤ��ު��B��ʻ��u��)��17%�F�ӨϥΤ㨺�̭�(Zanamivir)��ӻݧܥͯ��v���ֵo�g�u��11%(Kaiser
2000b)�C�ۤϡA�o�Ӽƾڤ����g�d�ҡC�t�~�@�Ӥj�����ʱ����{�ɬ�s�A���h��2,300�f�H�ѥ[�A�ӨϥΤ㨺�̭�(Zanamivir)���էO�Φw�����աA��̩ҥX�{�ֵo�g�Ʀr�]�t���h(Cole
2002)�C
�w��
�@�s�ꪺ�H������A�ҩ��F�㨺�̭�(Zanamivir)���T��w���y�P���ġC�b���礤�A���d���H���X�{�y�P�z�o��A���f�ħl�J�C��10�@�J�㨺�̭�(Zanamivir)�Φw�����A����4�ӬP���A�o�{��A�Τ㨺�̭�(Zanamivir)����67%�F�y�P�w���ĪG(6
% [34/554] �w�����էO�X�{�y�P�x��vs. 2 % [11/553] �㨺�̭�(Zanamivir)�էO�X�{�y�P�x��)�A�Φ�84%
�ਾ��X�{�o�N�x��(Monto
1999b)�C
�t�@���{�ɹ���A�A��2-5�H���a�x�A�����ܤ֦��@��5?�j�p���C�����䤤�@�Ӯa�x�����w���y�P��A���a�H�ݪA��10�@�J�C��㨺�̭�(Zanamivir)�Φw�����A����10��C���G��ܡA�A�Τ㨺�̭�(Zanamivir)���a�x���u��4%�X�{�s�y�P�ӮסF�ۤϡA�A�Φw�������a�x�t���h��19%�X�{�s�y�P�ӮסC�A�Τ㨺�̭�(Zanamivir)���ҥX�{�x���ɶ���2.5��A���A�Φw���������u(5-7.5��)
(Hayden
2000)�C�ӥt�@��s�A�P�ˬO�A�Τ㨺�̭�(Zanamivir)��A���֦]��IJ�y�P�����Ӯצӱw�W�y�P�����|(Kaiser
2000)�C
���@��s���G��ܡA�A�Τ㨺�̭�(Zanamivir)���a�x���u��4%�X�{�s�y�P�ӮסF�ۤϡA�A�Φw�������a�x�t���h��19%�X�{�s�y�P�ӮסC������w���į�F81%�C�ӹ��ӧO���w���įప�F82%�A�ι��ҡB�A���y�P���w���į���O��78%��85%
(Monto
2002)�C
�ൣ
�b��s���礤�A����F�~�֤��G5-12���C�P�˪A�Τ㨺�̭�(Zanamivir)���o�q���ҥX�{�x������Ʈɶ����1.25��A�[�W��f�U�ɶ����u�Υ��Ī����q����(Hedrick
2000)�C
�]���A�㨺�̭�(Zanamivir)���ൣ�O�w���A�p�L�̯�A�Υ��C�ൣ�A�S�O�O�����H�U���A�q�`����ϥ�����㨺�̭����t�Ρe�]�A���వ�������q�ת��l�J�v�ιF��A�����y�p�l��y�t�]�Y�C��C�����������窺�̲z�Q�t�ס^�f�C�b�ʥF��i�q�פ��l�J�v�H�P�������Φ�M����q�@�סA�t�ĭ��Ҽ{���Į����p�ߤ��R�ൣ�ϥΧl�Ǿ�����O�C���u���Ω�ൣ���ɫJ�A�@�w�n�����H�ʷ��Ϊ`���A�����ϥΧl�Ǿ�(Relenza
2003)�C
�S�����p
���w����ʲO�ڲӭM�զ�f(acute lymphoblastic leukemia)
(Maeda
2002)�δ��i��L����F�ӭM����(allogeneic stem cell transplantation)
(Johny
2002)���w�̡A��A�Τ㨺�̭�(Zanamivir)�����q�n�S�O�B�z�C�b�ĤG�ӳ��i���X�A�㨺�̭�(Zanamivir)���|�X�{�r�ʤ����C��S���X�{�L�o���f�H�]�V�W�y�P���`���f�ҡC
�V�y�P�f�r
�b2000�~���i��L�����㨺�̭�(Zanamivir)��y�P�f�r���ѹ�����A�o�{�㨺�̭�(Zanamivir)���H9N2,
H6N1�δ��X�{�������P�VH5N1���f�r������ĥ�(Leneva
2001)�C
���ĩ�
�X�{���ĩʤ�������u���C�ثe����A�q�K�̬��ʪ��f�H�����j���o����ĩʪ��f�r�C�[�W�A�Ҧ��b�պޤ����o���ܤ㨺�̭�(Zanamivir)�f�r�����ʩʫܮz�C�w���H�{�Ѫ����ĩʬ��ܬO���Y�ǯf�r�ȸs(virus
subtype)���Ī����S���ʪ�(drug specific) (McKimm-Breschkin
2003)�C
�{�b�A�禳���Ҿڻ����b�{�ɮɡA���g�i�J���(neuraminidase inhibitors)�����A�|�ް_���P���������ī~�ؤι藍�P���������e��ܪ��f�r�C
�Ī��ۤ��@��
�㨺�̭�(Zanamivir)�O�z�L�f�ħl�J�A�ܤ��Ī������i�i�J��G�ءA�ҥH���G�O��ߪ��Ī��@�ܧC�Υ������Ī�����IJ�]�O���q���C�ѩ�㨺�̭�(Zanamivir)���Q�N�¤��ѡA��ܤ־��|�X�{�Ī��P�Ī��������ۤ��@��(Cass
1999b)�C�㨺�̭�(Zanamivir)�S���O���(substrate)�A�G���|�v�T��xŦ�L����(liver
microsomes)���ӭM���P450 (cytochrome P450�ACYP)�B�P�u�J(isoenzymes
�ACYP1A1/2, 2A6, 2C9, 2C18, 2D6, 2E1, and 3A4)
(Relenza 2003)�C �o�بS���z�װ�¦�����㨺�̭�(Zanamivir)�|�P��L�ƦX�����ͥN�¬ۤ��@��(Daniel
1999)�C
��ij�Ϊk
- �㨺�̭�(Zanamivir)�@��A�ΩH�Ψൣ(�ڷ�:12���ΥH�W�F����:7���ΥH�W)�]�V�W�y�P�ҤΤA�f�r(influenza
A and B)�A�X�{�ǫD�����ʫ�ʼx���A�o�f�ɶ�����2�ѡC
- �㨺�̭�(Zanamivir)����ij�Ω�w�����ʩI�l�D�e�f���w��(�p���ݩκC�ʪ���ʩI�l�D�f,COPD)�C
�㨺�̭�(Zanamivir) (Relenza?)�ݥΤf�ħl�J�A�]���Τf�A������i�Q�Ϋ�(bioavailability)�ܧC�CRelenza?���۹F�С]Rotadisk�^�����A�C�ӱ۹F�Чt��4����������w�n�A�C�Ӫw�n�t���㨺�̭�(Zanamivir)
5 �@�J(�å[�J20�@�J�ſ})�C�C�Ӫw�n�ϥγ��ݳz�L�@�ӽ��������A�ٺЦ��l�Ǿ�(Diskhaler)�C
���w�n�Q����A�㨺�̭�(Zanamivir)���b�Ů𤤡A�ѯf�H�f�ظg�l�Ǿ��l�J�餺�C�ܩ�A���h���Ī����q��F�I�l�D�A�����G�l��t�סC
�w�̥������H���ܪA�ġA�C�i���ܤ]���ϥΥܽd�C�ӹ��ൣ�w�̡A���Ӧb���H���ɤU�θ��H���ܪA�ġC
�o�ئҼ{��@��Ѧ~�H�ϥΧl�Ǿ�����O�C�N�����D�A���b��|�ئV73�f�H(�~�֤��G71-99��)���L�լd�A�o�{�j�����~�ѤH�h�ϥΧl�Ǿ��X�{���D�A�v�T�㨺�̭�(Zanamivir)�v������(Diggory
2001)�C
���q
���H��7���H�W�ൣ����ij���q��10�@�J�C��2��(�s��l�J��Ӫw�n)�A����5�ѡC
���{�Ĥ@��A���Įɶ��ܤֹj2�p�ɤ@���C�~��A�ɶ����j12�p�ɤ@���C
���w���ǯf�w�̵L�ݽվ���ľ��q(Cass
1999a)�C
�p�G�]�A�Τ㨺�̭�(Zanamivir)�A�ӥX�{�I�l�B�@���աA���ߨ�ϥΧֳt����X�i���ΰ���ϥΤ㨺�̭�(Zanamivir)�C
�`��
�ӼЦW�١GRelenza®
�Ī������G���g�i�J���
�s�y�ӡGGlaxoSmithKline
���ܡG�㨺�̭�(Zanamivir)�@��A�ΩH�Ψൣ(�ڷ�:12���ΥH�W�F����:7���ΥH�W)�]�V�W�y�P�ҤΤA�f�r(influenza
A and B)�A�X�{�ǫD�����ʫ�ʼx���A�o�f�ɶ�����2�ѡC
�зǪv�����q�G10�@�J�C��2��(�s��l�J��Ӥ��@�J���w�n)�A����5�ѡC
�зǹw�����q�G�b�j�h�ƪ���a�A�祼���H�㨺�̭�(Zanamivir)�@�w���@�ΡC
�Ī��ʤO�ǡG�ʤ����Q�ܤG�Q���㨺�̭�(Zanamivir)�|�z�L�f�ħl�J��F�ͳ��A�l�U���|�d�b�|��A�j��4-17%�Ķq�Q�H��l���C�b1-2�p�ɤ��Ī��b��G���F�̰��@�סC��J���H�X�Q����(<
10 %)�C�㨺�̭�(Zanamivir)�̫�q���G���ƥX�A���Хb�I��2.5-5.1�p�ɡC
ĵ�i�G����ij�Τ㨺�̭�(Zanamivir)��w�����ʩI�l�D�e�f���w��(�p���ݩκC�ʪ���ʩI�l�D�f,COPD)�C
�ۤ��@�ΡG�ھڦb�պޤ��������s�A���p�㨺�̭�(Zanamivir)���|�ް_�����Ī��ۤ������C
�Ƨ@�ΡG�㨺�̭�(Zanamivir)�ݸ��w���Ī��A�j��W��ް_�I�l�D���}���������|�ܲӡC
�w�̸�ơG�ϥΤ㨺�̭�(Zanamivir)�@���v�����Ϋ�A�S�����y�P�ǬV�L�H�����|��֡C
�禳�ް_���j�˪����|�A�S�O�O�w�����ʩI�l�D�e�f���w�̡C�p���I�l�D�x���[��(�p�ݮ�B�I�l�x���Φ����j�˥���)�A���ߨ谱�Τ㨺�̭�(Zanamivir)�ç�����ˬd�C�w�����ݩκC�ʪ���ʩI�l�D�f(COPD)���w�����S�O�`�N�A�Φ��ϥΧֳt�����X�i�����ݭn�C
�p�w�̻ݦP�ɨϥΤ㨺�̭�(Zanamivir)�Χֳt�����X�i�����ܡA��ij���ϥΧֳt�����X�i���~�l�J�㨺�̭�(Zanamivir)�C
�J���Ī��̦n��25�X C (77�X F)�A�u�{�i�b��b15�X��30�X C (59�X to
86�X F)�����C
���p���ӷ��G
����G http://influenzareport.com/link.php?id=5
|

